This site uses cookies to provide you with a more responsive and personalized service. By using this site you agree to the Privacy Policy and Terms of Use.
I accept
Our Technology Platform
Early cancer vaccines include whole-cell formulations, which operate on the principle that you should vaccinate with what you want to develop protection against. Such vaccines have been widely tested in various cancers and their advantages described but have not yet managed to pass clinical trials. Antigenic Essence technology offers the possibility to revitalize the field of whole-cell-based vaccination, as the essence comprises the entire diversity of native cellular antigens. At the same time, the technology allows for precise control and purposeful change of essence composition as well as purification of essence from ballast cellular substances and also addresses issues of major histocompatibility complex restriction. Antigenic Essence technology makes it possible to update many cellular vaccines that have already been developed, as well as to develop new ones, therefore introducing a new direction for anticancer vaccination research.
Antigenic Essence Concept
The concept of antigenic essence arose from that of cellular vaccination, the driving principle of which is to vaccinate with what you want to destroy by vaccination. Despite the persistent failure of vaccines based on whole tumor cells, the development of such vaccines continues to occupy a leading position along with those based on tumor-associated antigens. Cells are a natural source for the entire diversity of native antigens, so the concept of antigenic essence takes advantage of this while also minimizing the limitations associated with the use of whole cells.
The first assumption of antigenic essence is that only antigens presented on the surface of the cell are targets for vaccination, while those presented inside the cell can and should be neglected. This is based on the fact that only antigens on the cell surface are available to the immune system; the plasma membrane of living cells is impermeable to cytotoxic elements of the immune system that recognize antigens, be they antibodies or T lymphocytes. This does not contradict the fact that some intracellular antigens can be presented by MHC on the cell surface and offer suitable targets for vaccination; indeed, some such antigens are currently undergoing clinical trials. On the other hand, extracellular antigens are produced inside cells and can therefore always be found there as well. The point is not where the antigens can be found, but that they function as targets for the immune system only when presented on the cell surface. That is, the target pool of antigens is located precisely on the cell surface, and they form an actual profile of the antigenic properties of living cells.
Given that the target pool of antigens is presented on the cell surface and the cell wall is impermeable to macromolecules, the second assumption of antigenic essence is that the target pool of antigens can be collected by treating living cells with protease (see picture). Despite the apparent simplicity of this approach, this is not a trivial process, and the implementation in mammalian cells became possible only with advances in proteomics. When a highly purified protease is used, cytolytic activity is low, thus allowing the treatment of live cells without violating the permeability of their membranes. The use of proteomics-grade protease makes it possible to obtain just the pool of antigens from the cell surface without contamination by intracellular content. Furthermore, the obtained antigens are not overridden by impurities or autolytic fragments of the protease itself. Last but not least, the obtained antigens can be analyzed by mass spectrometry to establish essence composition, and this information can be used to fine-tune the design of cancer vaccines and validate their quality.
The first assumption of antigenic essence is that only antigens presented on the surface of the cell are targets for vaccination, while those presented inside the cell can and should be neglected. This is based on the fact that only antigens on the cell surface are available to the immune system; the plasma membrane of living cells is impermeable to cytotoxic elements of the immune system that recognize antigens, be they antibodies or T lymphocytes. This does not contradict the fact that some intracellular antigens can be presented by MHC on the cell surface and offer suitable targets for vaccination; indeed, some such antigens are currently undergoing clinical trials. On the other hand, extracellular antigens are produced inside cells and can therefore always be found there as well. The point is not where the antigens can be found, but that they function as targets for the immune system only when presented on the cell surface. That is, the target pool of antigens is located precisely on the cell surface, and they form an actual profile of the antigenic properties of living cells.
Given that the target pool of antigens is presented on the cell surface and the cell wall is impermeable to macromolecules, the second assumption of antigenic essence is that the target pool of antigens can be collected by treating living cells with protease (see picture). Despite the apparent simplicity of this approach, this is not a trivial process, and the implementation in mammalian cells became possible only with advances in proteomics. When a highly purified protease is used, cytolytic activity is low, thus allowing the treatment of live cells without violating the permeability of their membranes. The use of proteomics-grade protease makes it possible to obtain just the pool of antigens from the cell surface without contamination by intracellular content. Furthermore, the obtained antigens are not overridden by impurities or autolytic fragments of the protease itself. Last but not least, the obtained antigens can be analyzed by mass spectrometry to establish essence composition, and this information can be used to fine-tune the design of cancer vaccines and validate their quality.
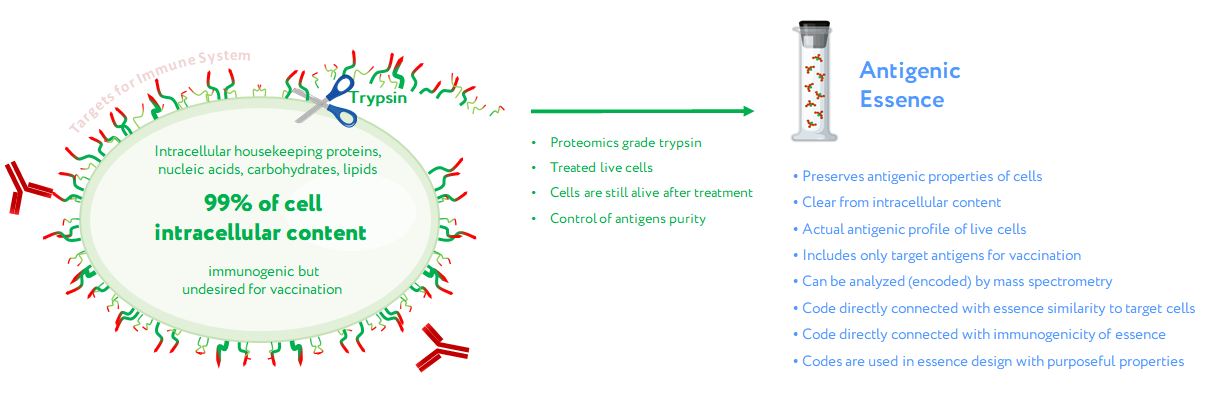
Concept of antigenic essence. Actual antigenic properties of live cells are defined by a pool of antigens presented on the cell surface. Intracellular content is considered noise to be excluded from the essence. After washing away traces of culture medium, cells are treated with a purified protease. Released fragments of the cell surface proteins are collected, analyzed by mass spectrometry, and used for vaccination instead of whole cells.
Ref.: Cancers. 2021; 13(4):774.
Ref.: Cancers. 2021; 13(4):774.
